RELEUKO® from Amneal Biosciences
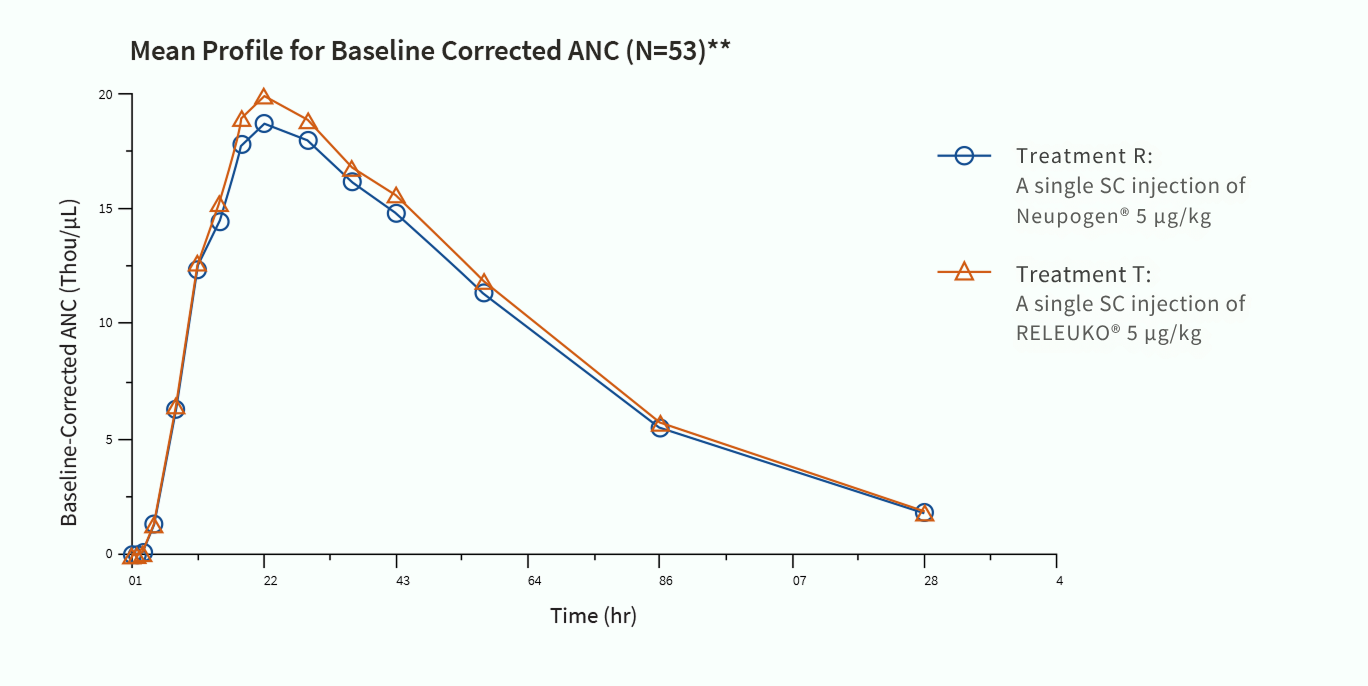
RELEUKO® (filgrastim-ayow) injection is biosimilar to Neupogen® (filgrastim) and made entirely in the United States.1
Filgrastim is a recombinant granulocyte colony stimulating factor (rG-CSF). It promotes neutrophil production in appropriate patients.