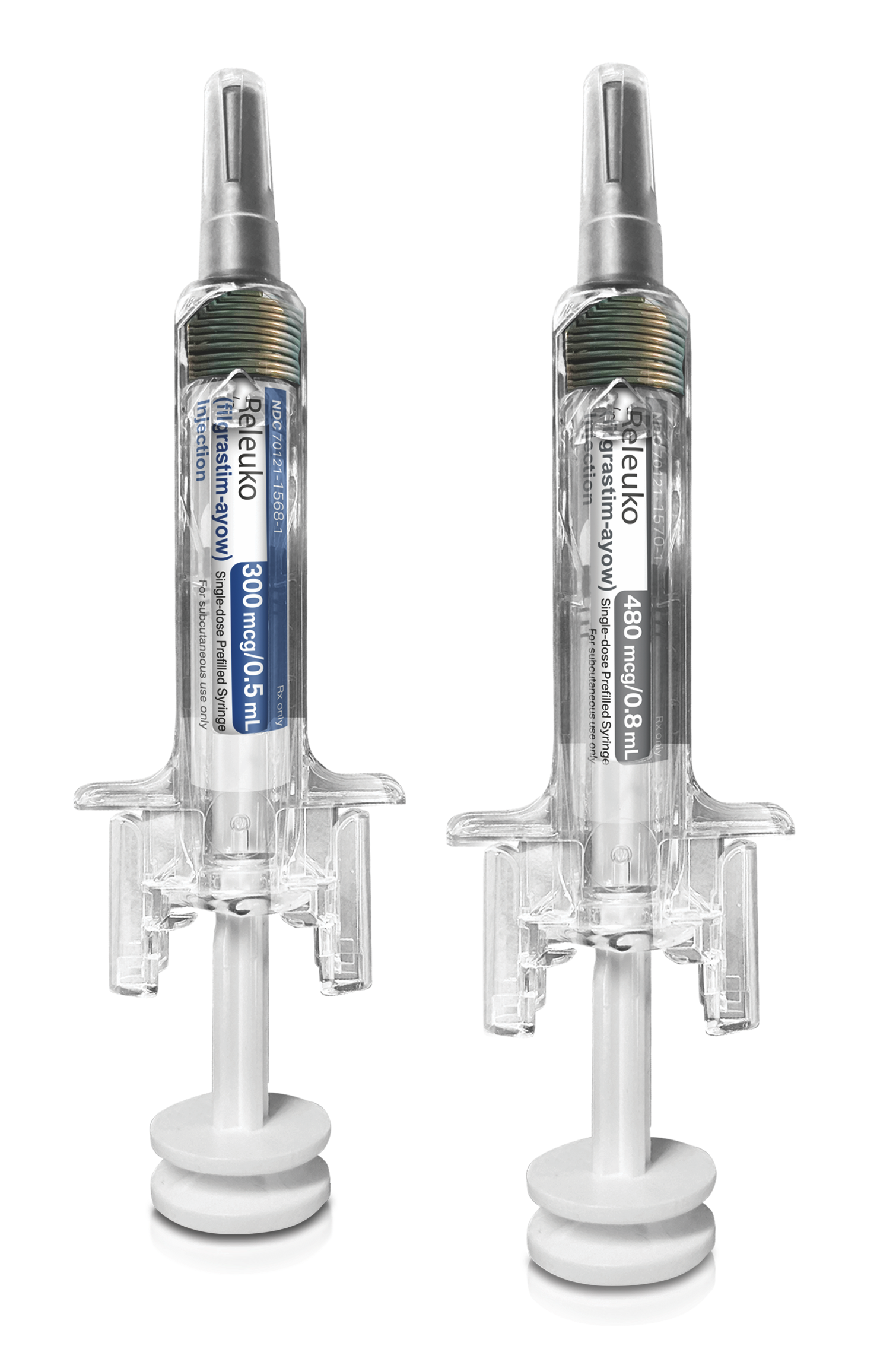
Coding Information
| HCPCS Code | Descriptor | |
|---|---|---|
| Q5125 | Injection, filgrastim-ayow, biosimilar, (releuko), 1 microgram |
| HCPCS Code | Q5125 |
|---|---|
| Descriptor | Injection, filgrastim-ayow, biosimilar, (releuko), 1 microgram |
Centers for Medicare & Medicaid Services. Month 2022 Update of the Hospital Outpatient Prospective Payment System (OPPS). Updated October 10, 2022.